elevating engagement. ensuring compliance.
With our best-in-class Titanium solutions and dedicated support, we are committed to helping your organization achieve compliance excellence while driving sustainable success in the dynamic life sciences industry.

WHO WE ARE
A Trusted Leader in Life Sciences
We offer a wide range of innovative technology solutions and services, with a comprehensive suite of services and solutions tailored to meet the diverse needs of our clients. With cutting-edge technology and a dedicated team of experts, QPharma will help you achieve compliance excellence and drive sustainable success in the ever-evolving landscape of the life sciences industry.
Powered by

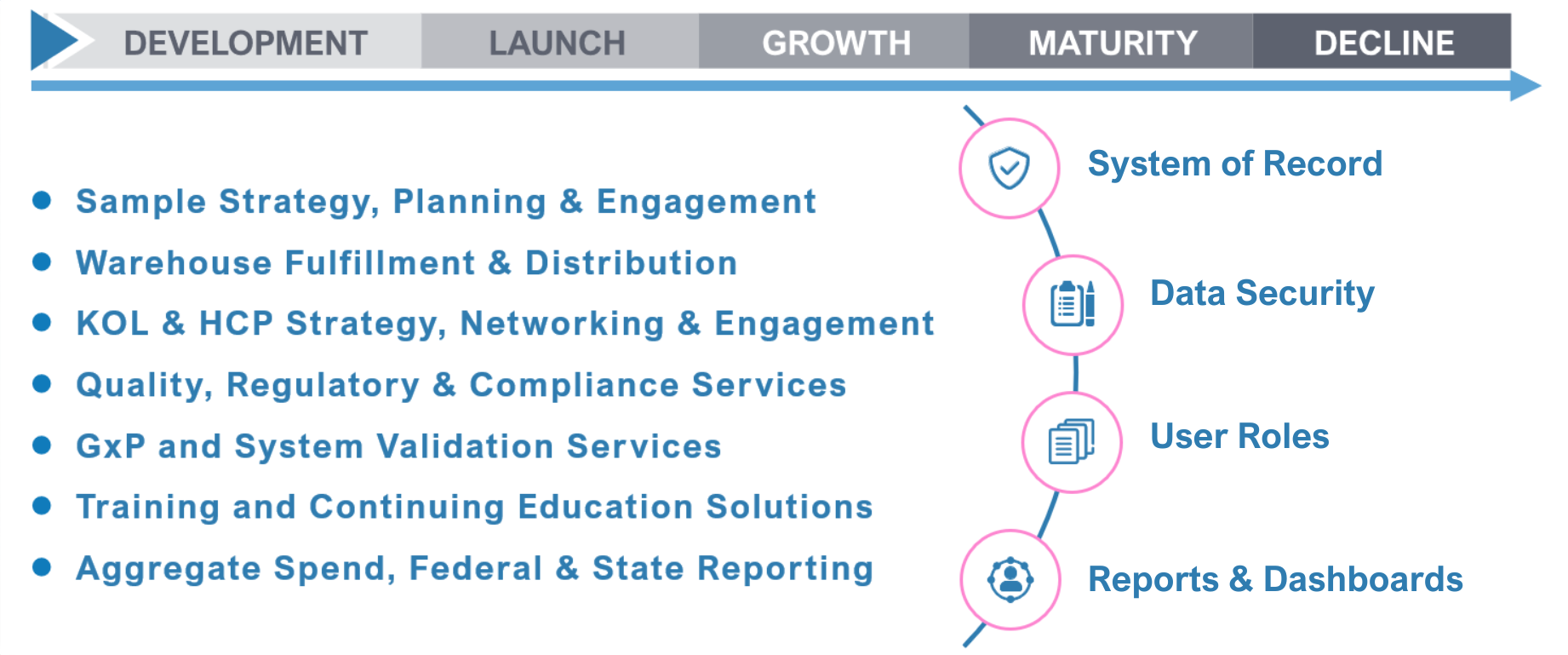
Titanium offers a comprehensive range of applications to ensure your sample management program remains compliant, efficient, and streamlined. Our cloud-based, SaaS platform delivers unparalleled benefits by integrating advanced technologies specifically designed to meet PDMA and 21 CFR Part 11 requirements.
From Launch to Legacy: QPharma’s Titanium Solutions Deliver
Connect with a business partner to learn how QPharma can help your
Who We Serve
What Our Clients Have To Say About Us
QPharma's IT team is innovative and proactive, always creating tailored solutions like custom dashboards and streamlined ordering processes. Their account managers are so familiar with my program that I can confidently take time off, knowing my department is in good hands. During the lockdown, they were instrumental in transitioning us to a direct-to-practitioner model. Their support, from inventory management to program updates, has been invaluable.
QPharma's IT team is innovative and proactive, always creating tailored solutions like custom dashboards and streamlined ordering processes. Their account managers are so familiar with my program that I can confidently take time off, knowing my department is in good hands. During the lockdown, they were instrumental in transitioning us to a direct-to-practitioner model. Their support, from inventory management to program updates, has been invaluable.
We wholeheartedly recommend the QPharma MedStart drug sample program to any healthcare institution seeking innovative technology solutions."

Why Choose QPharma?

Industry Leading Solutions
Unparalleled Compliance Expertise
Our deep understanding of PDMA compliance and industry best practices ensures adherence and support in meeting regulatory requirements.
Tailored to Your Needs
Titanium is configurable and scalable, allowing customization to meet your specific business needs, whether you require standard, ad-hoc, or custom reporting options.
White Glove Customer Service
We prioritize building solid partnerships with our clients, providing personalized and responsive support throughout our relationship.
What’s New
The latest news, insights, and product info from the QPharma team.

How to Choose a Compliant Life Sciences Technology Partner
Learn what qualities to look for in a life sciences technology partner to help you choose a provider that aligns with your needs and industry demands.

QPharma Unveils QPharmaRx: Revolutionizing Health Care Provider Engagement with Enhanced Sample Ordering and Portal Options
MORRISTOWN, N.J.–(BUSINESS WIRE)–QPharma®, a leader in compliance and brand support services for the pharmaceutical industry, proudly announces the integration and rebranding of its Ti Portal product as QPharmaRx™, offering clients enhanced flexibility and advanced features for healthcare provider (HCP) engagements.

QPharma Launches New Website Celebrating 30 Years of Compliance and Innovation
MORRISTOWN, N.J.–(BUSINESS WIRE)–QPharma is thrilled to unveil our new website, highlighting 30 years of expertise in compliance and cutting-edge technology. Our Titanium platform is tailored to help pharmaceutical clients effectively identify and engage with targeted healthcare professionals (HCPs), ensuring optimal patient health. Discover how our advanced solutions and deep industry experience can elevate your HCP medical and commercial engagement strategies.











